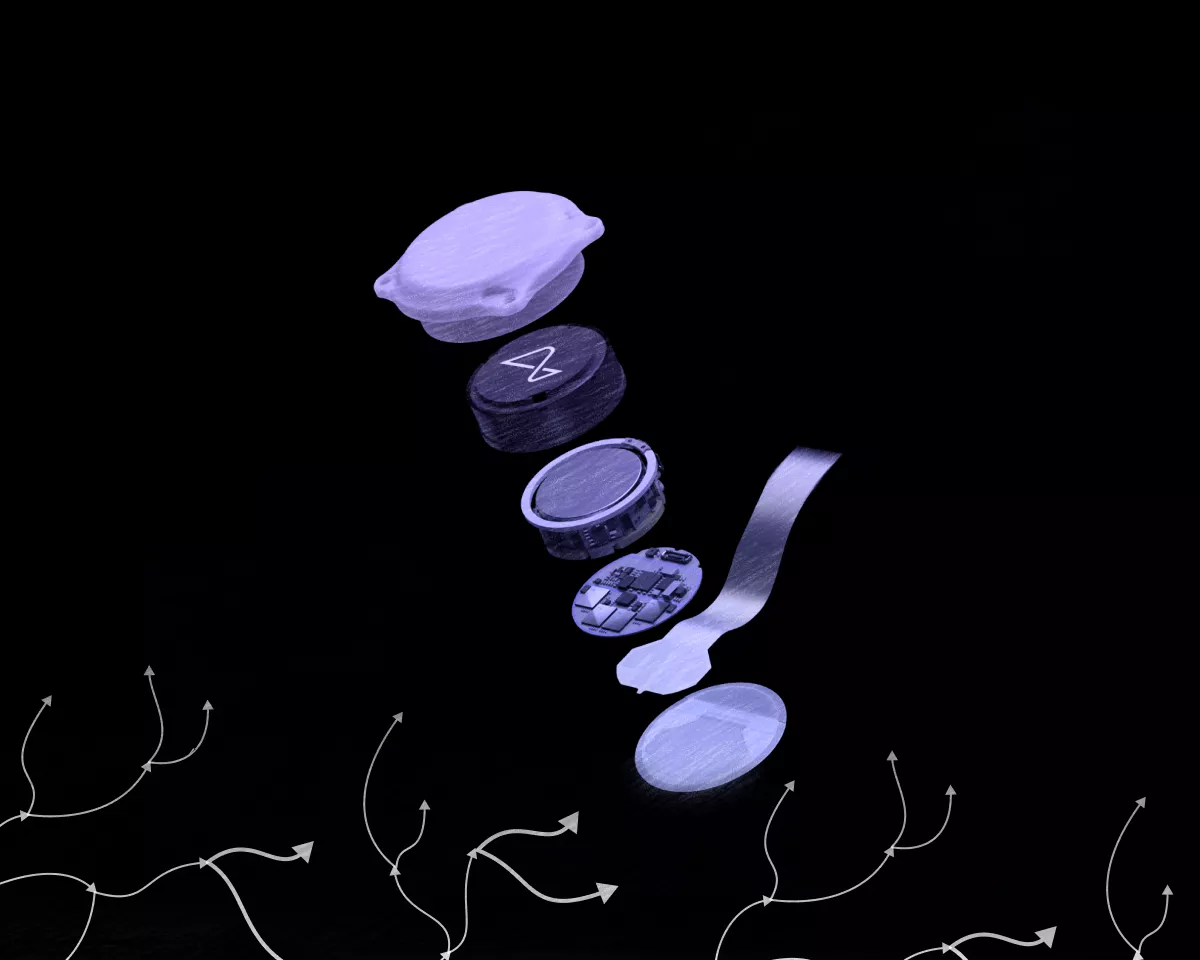
Canada’s Ministry of Health has given the green light for Neuralink to begin clinical trials of its neural chips. The company, founded by Elon Musk, has announced the recruitment of volunteers.
🇨🇦 We’re happy to announce that Health Canada has approved the launch of our first clinical trial in Canada! Recruitment is now open.
If you have quadriplegia due to ALS or SCI, you may qualify. Visit our Patient Registry to learn more and apply.https://t.co/5BySJABkkO
— Neuralink (@neuralink) November 20, 2024
One of Canada’s largest research and medical centres, University Health Network (UHN), announced that its Toronto Western Hospital will be the first outside the United States to implant Neuralink’s wireless brain-computer interface (BCI).
The institution conducts research in neurology, psychiatry, genetics, and other medical disciplines, developing new treatments and technologies.
“We are incredibly proud to be at the forefront of this research progress in neurosurgery. […] As the first and exclusive surgical centre in Canada for this procedure, we will continue to shape the future of neurological care […],” stated UHN President and CEO Kevin Smith.
The CAN-PRIME study aims to “assess the safety of the implant and surgical robot, the functionality of the BCI, and the potential to enable individuals to control external devices with their thoughts.”
“This landmark surgery has the potential to transform and improve outcomes for patients who previously had limited options,” commented CAN-PRIME study leader Andres Lozano.
In September, Neuralink began seeking volunteers for testing the Blindsight implant, which aims to restore vision.
Previously, the company reported on the achievements of a second patient who had a brain chip implanted.